
Water in Nature and Possible Industrial Uses
By
Roberto Triolo and Irene Ruffo
Dipartimento Di Chimica Fisica – Università di Palermo – Palermo (Italy)
Liquid water (H2O) is the most common substance in the world. Despite being apparently so simple, and therefore despite being considered an ordinary material, water is able to display a variety of different properties which make it the most important material in Nature. If we think how our day starts and how ends, how our days progress, we shall probably realize that most of our activities depend on water. The water molecule is very simple and small. Only three atoms make it: two Hydrogen atoms (H) and one Oxygen atom (O). However H and O are quite different atoms, the first one being very electropositive (has tendency to acquire a positive charge) and the other has tendency to acquire a negative charge. However, the

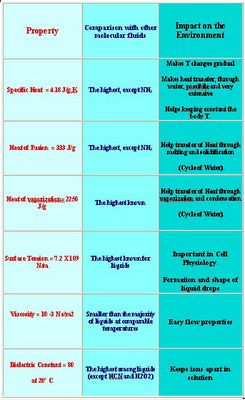
extraordinary complexity of its properties is also due to its size; on the other hand these properties seem to fit ideally into the requirements for life as no other molecule. All the most interesting properties seems to derive from the possibility of the opposite charges to interact generating a peculiar type of “bond”: the Hydrogen Bond (H-bond) which is responsible for the high specific heat, the unusually high heat of fusion and heat of evaporation, the high melting and boiling point, and the possibility of acting as solvent for polar molecules in its Normal Water conditions (NW) and as a more “organic type” solvent in Near Critical (NCW) and Super Critical (SCW) conditions. H-bonds are formed as the Hydrogen atom (the positive end of the molecule) is attracted from the Oxygen atom (the negative end of the closest water molecule). In these conditions a network of water molecules is formed. The net result is similar to having a macromolecule made by many single molecules held together. Water molecules are pretty much held together by these forces, so water surface is very resistant to penetration. Chemists call this property “Surface Tension”. In fact, any object, to penetrate the surface, must break this network of H-Bonds. A difficult task! It is well known that ice is lighter than liquid water: also this property is a consequence of the H-bonding, which create voids in the ice structure, and therefore a given volume of ice will weight less than the same volume of liquid water, which is heaviest at 4°C. Below and above this temperature water is lighter. The consequence is that lakes and oceans freeze only on the surface and not from the bottom. Without this property life would not be possible, neither the cycle of water would be possible. Water has a large heat of evaporation because, to evaporate, H-Bonds holding molecules together must be broken. This can be done, but only if large amounts of energy are used and so water has the largest heat of evaporation of any other material liquid at room temperature in Nature; water becomes solid at 0°C and boils at 100°C and therefore is liquid in the vast majority of the earth surface. When compared with the gas phase (water vapour vs. air), one may notice that water warms up more slowly and cools down more slowly than air. This makes life more comfortable for the living creatures, as our body temperature is basically constant, despite the seasonal and daily changes of the atmosphere. In the table shown in the next page some of the properties of water are reported together with their relevance to environmental issues. Finally the strong H-bonding which characterizes the solid form of water makes ice a very peculiar material. It is possible to slide on it, to skate on it, to make it act as heat sink, helping to maintain an average temperature ideal for life processes. Let’s now assume that we are breaking these bonds. When the H-bonds progressively break, water loses more and more the characteristics of solvent able to solubilize salts, acids and many of the basic chemicals necessary for life (“polar solvent”), and starts to become more like typical organic solvents (“apolar” solvents). So, while at room temperature and pressure water is a poor solvent for plastics, in Supercritical conditions (high temperature and high pressure) becomes a good solvent for plastics. In turn this means that water (which is not dangerous for the environment) may replace solvents like benzene and Fluoro-Chloro-Hydrocarbons which are used in many industrial processes and are very dangerous for the environment. In a sense we may consider water as a sort of universal solvent, whose properties can be changed at will by changing its physical conditions (T and P), so one may expect dramatic changes in the solvating properties of water, moving across the phase diagram. In view of these properties, it is possible to find applications for supercritical water (SCW) in eco-friendly processes. For example many organic molecules, as well as O2, N2, CO2, are miscible in all proportions with water under these conditions. This makes SCW a good medium for combustion reactions , while it may dissolve organic materials, like waste materials, garbage etc. This opens a variety of possible processes.
Reactions and Phase Equilibria in Near-critical Water (NCW)
In the temperature range of 250 to 350 °C (near-critical) water is an environmentally-benign solvent for a wide variety of manufacturing processes in the chemical, petrochemical, pharmaceutical, and plastics industries.

In general NCW has the following advantages:
· Replaces many less desirable solvents, such as aromatic hydrocarbons and chlorinated compounds, reducing the environmental impact.
· Avoids the use of polluting mineral acids and hazardous catalysts.
· Permits the minimization or even the elimination of unwanted by-products.
· Provides better control of reactions which can be run homogeneously instead of heterogeneously.
· Facilitates closed processes by reuse of waste material.
· Provides simple separation after reaction.
Reactions and Phase Equilibria in Super-critical Water (SCW)
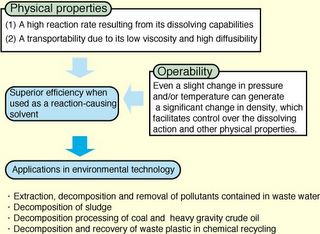
The chemical and physical properties of SCW, makes it an ideal medium for oxidation reaction of organic materials and their mixtures wit/without eteroatoms (halogens, sulphur, phosphorus, etc). The final products would be water and carbon dioxide for the organic portion, acids, bases and salts for the remaining portion. All these products can be easily separated and therefore pollution is very low. For this reason over the past decade, a major research effort has been focused on the destruction of toxic organics by means of total oxidation in SCW. The process is highly effective but there can be serious problems of corrosion associated with large scale waste destruction, so serious indeed that many chemists have been discouraged from even contemplating possible uses of SCW as a medium for chemical reactions.

Recently, however, there have been a number of reports which show that high temperature and supercritical water can be used constructively for reaction chemistry. The figure summarizes the main advantages of using SCW as reacting medium. Also a list of possible applications which begin to be explored is presented.
We wish to end this presentation reminding that Nature has already applied most of the “techniques” and “approaches” presented in this paper. Most geological fluids, in fact, contain water and carbon dioxide in supercritical conditions. This permits all the chemical transformations which give life to a variety of geochemical materials. It is for this reason that scientist have begun very recently to investigate not only NCW and SCW, but also mixtures of supercritical fluids, with water and CO2 as primary components.
This Lecture was delivered by the writers during the project meeting in Carini-Sicily-Italy- October 2003



















<< Home