EUTROPHICATION IN LAKES

Terms oligotrophic ("little food") and eutrophic ("well fed") were first used to describe soil fertility in northern Germany around 1905
This trophic classification of lakes was first developed largely on the basis of benthic invertebrates present in the deep water sediments. Some species were found to be more sensitive to low oxygen than others. This in turn could be related to classification as oligotrophic, mesotrophic, and eutrophic. Note that as originally proposed, only oligotrophic and eutrophic were defined.
1. OLIGOTROPHIC LAKES - tend to be deep with mean depths > 15 m and maximum depths > 25 m. Waters are transparent and have low density of plant life occurring at various depths. Nutrient supply is low in relation to the volume of water and dominant fish tend to be coldwater species such as lake trout.
2. EUTROPHIC LAKES - are shallow with mean depths <>MESOTROPHIC LAKES - a convenient term for lakes that are borderline between oligotrophic and eutrophic. They are intermediate with respect to nutrient supply, depth, biological productivity, water clarity, and oxygen depletion in the hypolimnion.
Lake Succession - Natural Eutrophication
Based on knowledge that oligotrophic lakes are deep and eutrophic lakes are shallow, it was inferred that lakes must evolve toward a condition of eutrophy over geological periods of time. Thus, the ultimate fate of lakes was to become filled with sediments and eventually supplanted by grassed or forests. Lakes accumulate sediments at an average rate of about 1 mm per year. Support for the idea that there is a successional process in lakes from oligotrophic to eutrophic was found through examinations of the fossil remains of indicator organisms in the sediments. The deepest sediments (oldest deposits) tended to have a greater abundance of organisms found in well oxygenated conditions and the shallowest sediments (most recently deposited) tend to have more organisms tolerant of low oxygen conditions. Despite some evidence to the contrary, there is widespread acceptance of the PROCESS OF NATURAL EUTROPHICATION. Natural eutrophication is complex, immeasurably slow (geological time periods), and, for all practical purposes, it is irreversible under a given set of climatic conditions. It is caused by the change in form and depth of the basin as it gradually fills in with sediment. To reverse natural eutrophication, you would have to scour out the lake basin; a formidable task under any circumstances and certainly not practical with current technology! Nutrient supply does not change, or if it does, it decreases as soils become exhausted.

Cultural Eutrophication
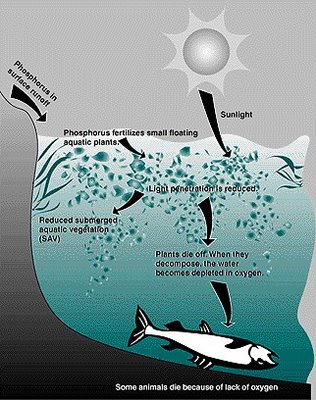
Human settlement in the drainage basin of a lake generally leads to clearing of the natural vegetation, the development of farms and cities. These activities in turn accelerate runoff from the land surface and increase the input of plant nutrients, i.e., the rate of nutrient supply is increased. Also, streams were convenient for disposing of household wastes and sewage, adding to the nutrient load in the receiving water body. The addition of plant nutrients stimulates the growth of algae and other plants which in turn stimulates fish and other organisms in the food web. This phenomenon is called CULTURAL EUTROPHICATION.

Cultural eutrophication is characterized by an intense proliferation of algae and higher plants and their accumulation in excessive quantities, which can result in detrimental changes in water quality and biological populations
The perceived negative effects of cultural eutrophication include reduced water transparency and excessive algal and plant growth, which is highly visible and can interfere with uses and aesthetic quality of water. One consequence of such growths may be taste and odour problems in drinking water. Ecological consequences include hypolimnetic anoxia due to algal decomposition and fish kills
In a few words: Cultural eutrophication is the rapid enrichment of water with nutrients derived from human activities. Main nutrients are P and N and they are derived from sewage, agricultural and livestock holding operations. This process is the result of an increase in nutrient supply to a constant volume of water, without any appreciable change in depth or form of the basin, as in natural eutrophication.
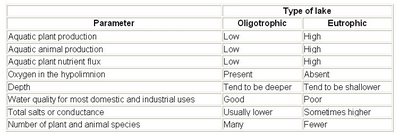 Table I. General Characteristics Frequently Used to Classify Lakes
Table I. General Characteristics Frequently Used to Classify LakesThe control of eutrophication is normally based on limiting aquatic plant nutrient input. Such control efforts must be directed toward the element which is currently limiting or can be made to limit algal growth in the body of water of concern. Domestic wastewaters represent potentially significant sources of nitrogen and phosphorus for the excessive fertilization of surface waters. Eutrophication control efforts are generally directed toward limiting the phosphorus content of domestic wastewaters by precipitation or co-precipitation treatment methods involving the use of aluminum or iron salts or lime. Other potentially significant sources of nitrogen and phosphorus include urban and rural storm water drainage and atmospheric inputs.
The control of nitrogen and phosphorus from urban and rural diffuse sources is a much more difficult task and will require the expenditure of large amounts of funds if excessive fertilization of natural waters it to be minimized to the greatest possible tent

IMPORTANT PARAMETERS
Physical parameters: Colour, Temperature, Turbidity and Odour.
Chemical parameters: pH, Electrical Conductivity (E.C), Total Solids (TS), Total Dissolved Solids (TDS), Total Suspended Solids (TSS), Total Hardness, Calcium Hardness, Magnesium Hardness, Nitrates, Phosphates, Sulphates, Chlorides, Dissolved Oxygen (D.O), Biological Oxygen Demand (BOD), Chemical Oxygen Demand (COD), Fluorides, Free Carbon-di-oxide, Potassium and Sodium.
Heavy metals: Lead, Copper, Nickel, Iron, Chromium, Cadmium and Zinc.
Biological parameters: The biological parameters involved the qualitative analyses of planktons (zooplankton and phytoplankton).
COLOUR
In natural water, colour is due to the presence of acids, metallic ions, suspended matter, plankton, weeds and industrial effluents. Colour is removed to make water suitable for general and industrial applications and is determined by visual comparison of the sample with distilled water.

pH
The pH of a sample of water is a measure of the concentration of hydrogen ions. The term pH was derived from the manner in which the hydrogen ion concentration is calculated - it is the negative logarithm of the hydrogen ion (H+) concentration. What this means to those of us who are not mathematicians is that at higher pH, there are fewer free hydrogen ions, and that a change of one pH unit reflects a tenfold change in the concentrations of the hydrogen ion. For example, there are 10 times as many hydrogen ions available at a pH of 7 than at a pH of 8. The pH scale ranges from 0 to 14. A pH of 7 is considered to be neutral. Substances with pH of less that 7 are acidic; substances with pH greater than 7 are basic.

The pH of water determines the solubility (amount that can be dissolved in the water) and biological availability (amount that can be utilized by aquatic life) of chemical constituents such as nutrients (phosphorus, nitrogen, and carbon) and heavy metals (lead, copper, cadmium, etc.). For example, in addition to affecting how much and what form of phosphorus is most abundant in the water, pH may also determine whether aquatic life can use it. In the case of heavy metals, the degree to which they are soluble determines their toxicity. Metals tend to be more toxic at lower pH because they are more soluble.
When pollution results in higher algal and plant growth (e.g., from increased temperature or excess nutrients), pH levels may increase, as allowed by the buffering capacity of the lake. Although these small changes in pH are not likely to have a direct impact on aquatic life, they greatly influence the availability and solubility of all chemical forms in the lake and may aggravate nutrient problems. For example, a change in pH may increase the solubility of phosphorus, making it more available for plant growth and resulting in a greater long-term demand for dissolved oxygen.
Generally, during the summer months in the upper portion of a eutrophic lakes, pH will range between 7.5 and 8.5. In the bottom of the lake or in less productive lakes, pH will be lower, 6.5 to 7.5, perhaps.
TURBIDITY
 Even relatively small amounts of wave action can erode exposed lakeshore sediments
Even relatively small amounts of wave action can erode exposed lakeshore sediments
High concentrations of particulate matter can modify light penetration, cause shallow lakes and bays to fill in faster, and smother benthic habitats - impacting both organisms and eggs. If light penetration is reduced significantly, macrophyte growth may be decreased which would in turn impact the organisms dependent upon them for food and cover. Reduced photosynthesis can also result in a lower daytime release of oxygen into the water. Effects on phytoplankton growth are complex depending on too many factors to generalize.
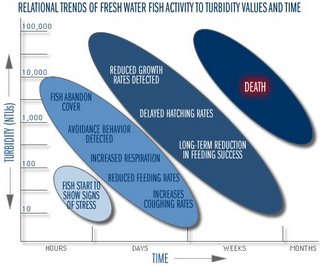
Schematic adapted from "Turbidty: A Water Quality Measure", Water Action Volunteers, Monitoring Factsheet Series.
Very high levels of turbidity for a short period of time may not be significant and may even be less of a problem than a lower level that persists longer. Turbidity is reported in nephelometric units (NTUs), but may also be measured in Jackson Turbidity Units (JTU). Waters with a turbidity level of > 5 NTU are not safe for recreational use or human consumption. Levels > 25 NTU cannot sustain Aquatic life.
The easiest and cheaper method to measure the water turbidity is using the Secchi Disk
SECCHI DISK

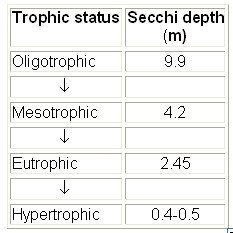
A Secchi disk is a circular plate divided into quarters painted alternately black and white. The disk is attached to a rope and lowered into the water until it is no longer visible. Secchi disk depth, then, is a measure of water clarity. Higher Secchi readings indicate clearer water. Lower readings indicate turbid or colored water. Clear water lets light penetrate more deeply into the lake than does murky water. This light allows photosynthesis to occur and oxygen to be produced. The rule of thumb is that light can penetrate to a depth of about 2 - 3 times the Secchi disk depth.

Taking a Secchi Disk reading
- Tie a wrist loop at the end of the rope so the rope end does not accidentally drop into the water when the disk is lowered.
- Lower the disk into the water until the disk just disappears from sight.
- Record the amount of rope submerged (i.e., note the point where the rope and water line meet).
- Raise the disk slowly until the disk just becomes visible and record this depth as well.
- Average the two recorded values. This average is called the limit of visibility.
If the disk hits the bottom before dropping out of sight, note this observation and record the bottom depth.
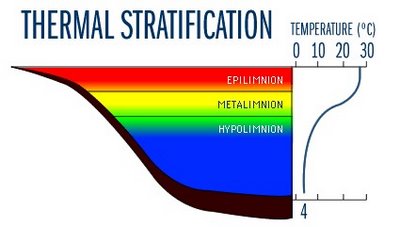
TEMPERATURE
Most aquatic organisms are poikilothermic - i.e., "cold-blooded" - which means they are unable to internally regulate their body temperature. Therefore, temperature exerts a major influence on the biological activity and growth of aquatic organisms. To a point, the higher the water temperature, the greater the biological activity. Fish, insects, zooplankton, phytoplankton, and other aquatic species all have preferred temperature ranges. As temperatures get too far above or below this preferred range, the number of individuals of the species decreases until finally there are few, or none.

Temperature is also important because of its influence on water chemistry. The rate of chemical reactions generally increases at higher temperature, which in turn affects biological activity. An important example of the effects of temperature on water chemistry is its impact on oxygen. Warm water holds less oxygen that cool water, so it may be saturated with oxygen but still not contain enough for survival of aquatic life.
Thermal pollution (i.e., artificially high temperatures) almost always occurs as a result of discharge of municipal or industrial effluents.
PHOSPHATES
Phosphorus is one of the key elements necessary for growth of plants and animals. Phosphorus in elemental form is very toxic and is subject to bioaccumulation.
Their presence in water is due to detergents, fertilizers and biological processes. They occur in solution in particles or as detritus. They are essential for the growth of organisms and a nutrient that limits the primary productivity of the water body. Inorganic phosphorus plays a dynamic role in aquatic ecosystems; when present in low concentration is one of the most important nutrients, but if the rainfall can cause varying amounts of phosphates to wash from farm soils into nearby waterways. Phosphate will stimulate the growth of plankton and aquatic plants which provide food for fish. This increased growth may cause an increase in the fish population and improve the overall water quality. However, if an excess of phosphate enters the waterway; algae and aquatic plants will grow wildly, choke up the waterway and use up large amounts of oxygen. It is calculated by the stannous chloride (SnCl2) method.

NITRATES
Nitrogen is one of the most abundant elements. About 80 percent of the air we breathe is nitrogen. It is found in the cells of all living things and is a major component of proteins. Inorganic nitrogen may exist in the free state as a gas N2, or as nitrate NO3-, nitrite NO2-, or ammonia NH3+. Organic nitrogen is found in proteins and is continually recycled by plants and animals
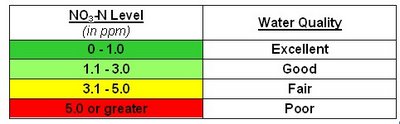
Nitrates are the most oxidized forms of nitrogen and the end product of the aerobic decomposition of organic nitrogenous matter. The significant sources of nitrates are chemical fertilizers from cultivated lands, drainage from livestock feeds, as well as domestic and industrial sources. Natural waters in their unpolluted state contain only minute quantities of nitrates. The stimulation of plant growth by nitrates may result in eutrophication, especially due to algae. The subsequent death and decay of plants produces secondary pollution. They can be measured by the phenoldisulphonic method.

(This work was presented by the Greek team in the project meeting in Ioannina (27-04-2006))


















0 Comments:
Post a Comment
<< Home